
Philippe Cotelle
Axes de recherche
Design of TEAD ligands for regenerative medicine (activators) and the treatment of cancers (inhibitors)
Hippo signaling pathway is a conserved regulator of organ size which is composed of a core kinase cascade. Phosphorylation of the downstream effectors YAP or TAZ changes their subcellular localization from the nucleus (unphosphorylated) to the cytoplasm (phosphorylated) (Figure 1). Unphosphorylated YAP and TAZ translocate to the nucleus and activate several nuclear transcriptional factors included TEADs. The activation of TEADs induces the expression of several Hippo pathway target genes including CTGF, Cyr61, Axl, Ankrd1 and Survivin (Birc-5).

Figure 1: A model of the Hippo signaling pathway in mammalian cells (from Piccolo et al. Physiol Rev. 2014)
Mammals express four TEAD (Transcriptional enhancer factor TEF with TEA/ATTS domain) genes, TEAD1-4. TEADs are broadly expressed but have tissue specific expressions which indicates tissue-specific roles. TEADs are the final effectors of the Hippo signaling pathway. Their functions are mainly mediated by their interactions with the nuclear coactivators (Yes Associated Protein) and its paralog TAZ (Transcriptional coactivator with PDZ-binding motif) (also called WWTR1).
YAP/TAZ and TEADs form a complex through the interaction of the N-terminal domain of YAP (and TAZ) and the C-terminal domain of TEAD. The N-terminal part of YAP (or TAZ) wraps around the ordered C-terminal domain of TEAD giving raise to the three interfaces of contact. Their respective importance in the binding are as follows: interface 3 (in red on Figure 1A) > interface 2 (in green) > interface 1 (in blue) (Figure 2). However, the minimal fragment of YAP or TAZ which gives a nanomolar range binding constant corresponds to interfaces 2 and 3. TEADs are palmitoylated in an internal hydrophobic pocket. This palmitoylation is required for the stability of TEAD, the interaction with YAP or TAZ and regulates the output of the Hippo pathway. This internal pocket clearly appears as the third druggable site of TEAD in purple (Figure 2B).
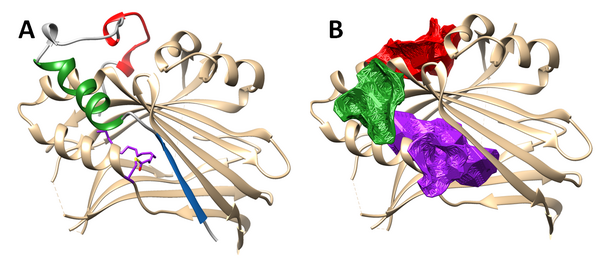
Figure 2: (A): The 3D-structure model built by superimposition of hYAP250-171-hTEAD1209-426 complex (PDB code 3KYS) and hTEAD2217-447 (PDB code 5EMV) with interface 1 (blue), 2 (green) and 3 (red); (B): The three predicted druggable sites of TEAD
YAP and TAZ are also deeply integrated in the Wnt pathway. The core of the Wnt pathway is to regulate the nuclear transducer b-catenin by a cytoplasmic destruction complex, consisting in Axin that interacts with other factors such as adenomatous polyposis coli (APC), glycogen synthase kinase-3 (GSK-3) and YAP or TAZ. In the absence of Wnt signaling, the destruction complex targets b-catenin to degradation through phosphorylation by GSK-3 and ubiquitination by b-TrCP, otherwise b-catenin migrates into the nucleus and interacts with TCF/LEF to promote embryonic stem cell self-renewal and intestinal homeostasis.
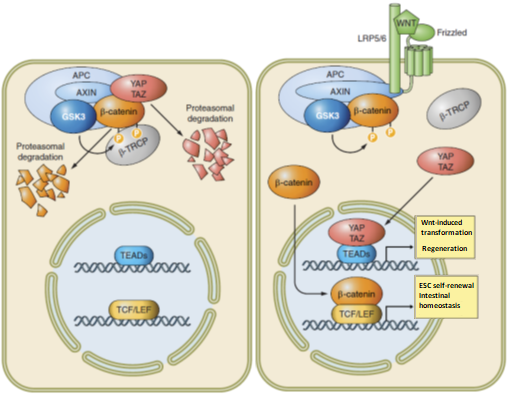
Figure 3: YAP and TAZ in the Wnt signaling pathway (from Piccolo et al. Physiol Rev. 2014)
In Huntington’s disease, these two pathways are dysregulated. While several reports are in favor of a decrease in nuclear YAP (and increase of P-LATS1 and P-MST1) in post-mortem human cortex with the implication of TEAD1, the role of the Wnt pathway is more controversial.
In addition, dysregulation of the Hippo pathway with resultant inhibition of TEAD transcriptional activity has been shown in many diseases including Alzheimer disease, Coloboma, Sveinsson’s chorioretinal atrophy and amyotrophic lateral sclerosis.
The aims of our project are:
1) to develop new TEAD ligands able either to activate or to inhibit YAP/TAZ-TEAD interactions. We focus our attention to the design of selective ligands for the treatment of specific disease (for example TEAD1 activators for Sveinsson’s chorioretinal atrophy or TEAD2/4 inhibitors for colorectal cancer) (Coll. Pr Jean-François Guichou, Montpellier).
2) to explore whether these activators could be beneficial to Huntington disease (coll. Dr Alexis Bemelmans, Pr Emmanuel Brouillet, Dr Sandrine Humbert) and other retinopathies (coll. Dr Muriel Perron).
3) to establish the role of the Hippo pathway in the control of neural stem/progenitor cells in tanycytes (coll. Dr Ariane Sharif).



